Skene's Gland Prostate Change On Testosterone Ftm

Article Menu
/ajax/scifeed/subscribe
Open Access Feature Paper Review
Inflammation and NF-κB Signaling in Prostate Cancer: Mechanisms and Clinical Implications
1
VIB-UGent Center for Inflammation Research, Unit of Molecular Signal Transduction in Inflammation, VIB, 9052 Ghent, Belgium
2
Department of Biomedical Molecular Biology, Ghent University, 9000 Ghent, Belgium
*
Author to whom correspondence should be addressed.
Received: 31 July 2018 / Revised: 24 August 2018 / Accepted: 27 August 2018 / Published: 29 August 2018
Abstract
Prostate cancer is a highly prevalent form of cancer that is usually slow-developing and benign. Due to its high prevalence, it is, however, still the second most common cause of death by cancer in men in the West. The higher prevalence of prostate cancer in the West might be due to elevated inflammation from metabolic syndrome or associated comorbidities. NF-κB activation and many other signals associated with inflammation are known to contribute to prostate cancer malignancy. Inflammatory signals have also been associated with the development of castration resistance and resistance against other androgen depletion strategies, which is a major therapeutic challenge. Here, we review the role of inflammation and its link with androgen signaling in prostate cancer. We further describe the role of NF-κB in prostate cancer cell survival and proliferation, major NF-κB signaling pathways in prostate cancer, and the crosstalk between NF-κB and androgen receptor signaling. Several NF-κB-induced risk factors in prostate cancer and their potential for therapeutic targeting in the clinic are described. A better understanding of the inflammatory mechanisms that control the development of prostate cancer and resistance to androgen-deprivation therapy will eventually lead to novel treatment options for patients.
1. Introduction
1.1. The NF-κB Family of Transcription Factors
Inflammation and cancer have been described in the medical literature for millennia [1], and there have been suggestions of a link between inflammation and cancer for centuries [2], but the significant overlap in molecular mechanisms between inflammation and cancer has only begun to be appreciated in recent decades [3,4]. Major players in both inflammation and cancer are the NF-κB transcription factors, which thus need to be tightly regulated [5]. The link between NF-κB and cancer was already apparent at its early discovery, since the oncogenic viral NF-κB family protein v-Rel was identified as a κB DNA binding transcription factor [6]. Many more viral proteins that induce inflammatory pathways also promote cancer development [7]. In addition, many mutations in NF-κB signaling proteins that cause dysregulated pro-inflammatory signaling also induce cancer [8].
The NF-κB family of transcription factors consists of different proteins (NF-κB1 (also known as p105), NF-κB2 (also known as p100), RelA (also known as p65), RelB and c-Rel) that are defined by their Rel homology domain (RHD), which is important for protein–protein interactions and assembly of an active transcription factor complex (Figure 1a). The transactivating (class II) NF-κB family members (RelA, RelB and c-Rel) are kept inactive by binding to the ankyrin repeat domain of inhibitor of NF-κB (IκB) family members, including p100, p105 and IκB proteins, which prevent NF-κB from entering the nucleus. Notable for p100 and p105 is that they can be processed into the p52 and p50 subunits, respectively, removing their 'inhibitory' ankyrin repeat domain. The p50 and p52 NF-κB (class I) subunits are DNA binding, but do not have trans-activation activity by themselves. NF-κB binds to specific DNA sequences as multiple homo- or heterodimers, with only dimers containing a class II subunit with a transactivation domain (TAD) being transcriptionally active. RelA and c-Rel can be active on their own as homodimers, whereas RelB depends on heterocomplexes with p50 or p52. The different NF-κB complexes are not redundant, and specific NF-κB complexes are activated via two major pathways known as the canonical and the non-canonical NF-κB pathway, respectively (Figure 1b). The standard canonical pathway for NF-κB activation relies on the canonical IKK (IκB kinase) complex consisting of IKKα, IKKβ and IKKγ (NEMO), and is best described for several pro-inflammatory stimuli such as tumor necrosis factor (TNF). Upon stimulation of cells, IKKβ phosphorylates IκBα, which leads to its subsequent ubiquitination and proteasomal degradation, thus releasing and allowing the p50/RelA complex to enter the nucleus. In certain conditions (e.g., downstream of certain viral receptors or in certain cancers), the IKKβ-related kinases IKKε and TBK1 can also activate NF-κB via IκBα, RelA and c-Rel phosphorylation [9], while IKK-independent mechanisms have also been reported (e.g., in response to DNA damage or hypoxia). In contrast to the critical role of IκBα in the canonical NF-κB pathway, the other IκB family members (IκBβ, IκBε, IκBζ, Bcl-3, IκBNS) seem to play more subtle roles (see below). The non-canonical NF-κB pathway, which is best known for its role in lymphoid organ development, is independent of IKKβ, but is regulated by the kinases NIK and IKKα. Notable for the non-canonical pathway is that it induces processing of the p100 pre-protein into the active p52 subunit. In the non-canonical pathway, p52 primarily activates NF-κB transcription, together with the transactivating RelB subunit (Figure 1b). The IKKα-related kinase IKKε can, however, also promote p52 transactivation by RelA [10].
The c-Rel NF-κB subunit is strongly associated with the development of many types of cancer [11]. In contrast to p50/RelA and p52/RelB, the regulation of c-Rel is less clear, but transcriptionally active complexes can occur as homodimers or as c-Rel/p50, c-Rel/RelA and c-Rel/p52 heterodimers [12]. In some cellular systems, RelB can act as an inhibitor of c-Rel-specific transcriptional responses [13], and c-Rel has also been suggested to be specifically negatively regulated by IκBε [14]. In contrast, IκBβ seems to promote NF-κB-dependent responses [15], especially c-Rel-dependent and RelA homodimer transcriptional activity [16,17].
NF-κB subunits do not exclusively interact with each other, and other "hybrid" active transcription complexes can also occur where DNA binding is provided by p50 or p52, but with transactivation by a different kind of protein. For example, the IκB family members Bcl-3 and IκBζ have transactivating activity and can form nuclear heterocomplexes with p52 or p50 to promote specific NF-κB-driven transcription [18]. Bcl-3 in complex with NF-κB is known to promote pro-survival and pro-proliferation transcription and suppress pro-inflammatory transcription after protein kinase C (PKC) activation by phorbol ester stimulation [19,20], which is important for the development of certain cancers.
1.2. Prostate Cancer: Role of Inflammation and Androgen Hormones
The prostate is a slightly bigger than walnut-sized gland (~20 g) in men, which produces the alkaline part of the seminal fluid to protect sperm against the acidic pH environment in the vagina. Prostate cancer is the most commonly occurring form of cancer in men, with 40–70% of men between 60 and 70 years old in Western countries dying from other causes have prostate cancer identified during autopsy, and the frequencies are even higher at more advanced ages [21]. A smaller (~5 g) corresponding organ, the female prostate (aka Skene's gland), is actually also present in females of various species, but its function is unclear, and the low awareness of this organ in women might lead to misdiagnosis of diseases associated with it [22,23]. Cancer originating from the female prostate appears very similar to male prostate cancer, but it is most likely extremely rare [24]. The only non-human animal known to also spontaneously develop prostate cancer is dogs, where the disease is very similar to that of humans, except that it is rarer and more aggressive [25]. The similarities between dog and human prostate cancer make dogs good animal models for the disease [26].
The prevalence of prostate cancer in men might be higher in Western countries due to being a comorbidity to obesity-related diseases like insulin resistance and constant inflammation [27,28,29,30]. Inflammatory signals in the prostate will enhance proliferation of otherwise normal epithelial cells [31], which could be a starting point for cancer development. Further indications for an important role of inflammation in prostate cancer initiation is provided by genetic analyses that have found associations between inflammation-related genes and prostate cancer risk [32]. Inflammatory signals and insulin-like signaling are, however, also involved in normal prostate growth and development [33]. Despite prostate cancer typically being a slowly developing and benign form of cancer [34], the high frequency of cases in the male population still results in a significant number of malign cases, making prostate cancer the 2nd leading cause of death by cancer in males in the US [21,35]. Malignant prostate cancer cells migrate to lymph nodes and can end up in bone, brain, liver and lungs, and liver metastasis, in particular, seems associated with poor survival [36,37].
Just as breast cancers are usually addicted to the female sex hormone estrogen, initially, prostate cancer typically also starts addicted to the male sex hormone androgen [38,39]. One treatment of prostate cancer has thus been to limit the amount of androgen, which could be achieved by castration [40], but tumor cells can start to produce their own androgens and become castration-resistant [41]. Prostate cancer in dogs is typically androgen-independent and aggressive, but castration prior to development of prostate cancer is still protective, indicating a role for androgens in the early stages also in dog prostate cancer [25]. Castration induces cell death and inflammation in the prostate, which needs to be controlled by macrophages [42], but these immunosuppressive myeloid cells can also contribute to the development of castration-resistant prostate cancer [43]. Noteworthy, induction of endogenous androgen biosynthesis in prostate cancer cells is at least partially driven by NF-κB, and might thus also be promoted by inflammation induced by castration [44].
The rare cases of female prostate cancer, together with reports of the development of androgen-independent aggressive forms of prostate cancer in transgender women up to 30–40 years after male-to-female corrective surgery (including castration), challenge the concept of androgens being required in the initial phases of prostate cancer [24,45,46]. In contrast, animal experiments with androgen injections in females have revealed growth of the female prostate, coupled with elevated secretory activity and serum prostate-specific antigen (PSA) levels [47]. These animal experiments also seem to reflect what happens in humans, since elevated PSA levels have also been observed in transgender men (female-to-male) undergoing androgen treatment [48]. There are, however, currently no reports of elevated female prostate cancer risk in transgender men after androgen treatments compared to cisgender (i.e., sex at birth is the same as the identified gender [49]) women. The rare cases of typically sex hormone-dependent cancer types in the "wrong" sex (e.g., cis-female prostate cancer and cis-male breast cancer) could perhaps also provide further insights on sex hormone-independent or alternative variations of sex hormone dependency in the early development of these cancer types. For example, the androgen receptor (AR) and the estrogen receptor (ER) β seem to promote male breast cancer, whereas both sex hormone receptors seem to inhibit female breast cancer [39].
Androgen signal deprivation is nowadays typically achieved by AR antagonists that might also be efficient against some cases of castration-resistant prostate cancer that depend on alternative androgen metabolism [50]. A remaining problem, however, is that prostate cancer also evolves into an AR-independent cancer after long-term treatment with AR antagonists, and AR antagonist-resistant cells may show elevated metastatic growth [51,52]. Identification of the mechanisms responsible for the development of androgen deprivation resistance is thus an important challenge in devising durable prostate cancer treatment options. Potentially important pieces of the puzzle for both prostate cancer androgen deprivation resistance and prostate cancer initiation are pro-inflammatory signals, which can be prostate cancer cell intrinsic, come from associated immune cells, or come from an interaction between the two [53,54,55,56,57,58]. Because of the many links between inflammatory signals and the development and malignancy of prostate cancer, targeting NF-κB has been suggested as a promising therapeutic option [59].
2. NF-κB in Prostate Cancer
2.1. NF-κB in Prostate Cancer Cell Survival and Proliferation
Several studies have demonstrated that NF-κB promotes cell survival, proliferation and invasion in prostate cancer [60,61,62]. Of the different NF-κB family subunits, especially p52 [63] has been shown to be important, but the transactivating subunits RelA [64], RelB [65,66] and c-Rel [67] have all also been implicated in prostate cancer. Notably, Bcl-3 is also important for prostate cancer cell survival during chemotherapy [68]. The development of treatment resistance is thought to depend on so-called cancer stem cells [69], and an AR-negative prostate cancer stem cell population with constitutive NF-κB activity was recently discovered [70]. It is possible that the AR-negative prostate cancer cells grow out of this stem cell population during androgen deprivation therapy, which means that NF-κB inhibition could be a promising target to prevent the evolution of AR-negative prostate cancer cells. NF-κB plays a key role in the development of castration or AR antagonist resistance, and combination treatments with NF-κB and AR inhibitors have shown promising results [71,72]. The role of NF-κB in development of castration-resistant prostate cancer has also been verified in mouse genetic models with constant NF-κB activation in prostate cancer cells [73]. A further indication for the role of NF-κB in prostate cancer proliferation is the observation that the NF-κB-inhibiting drug Aspirin and its active metabolite salicylic acid (SA) are both capable of significantly inhibiting growth of the androgen-independent prostate cancer cell line DU-145 [74]. Aspirin could, however, also act on prostate cancer via cyclooxygenase inhibition (COX) [75], which drives prostaglandin biosynthesis, and prostaglandins are important for prostate cancer cell growth [76,77,78,79]. Since free SA also shows inhibitory effects, and free SA cannot inhibit COX, the most likely explanation is, however, a direct effect of SA on NF-κB signaling at many different levels [80]. Interestingly, SA has also been shown to directly bind high mobility group box protein 1 (HMGB1), whose overexpression in prostate cancer is closely associated with the proliferation and aggressiveness of tumor cells, and which is known to promote the epithelial-to-mesenchymal transition in prostate cancer PC3 cells via the receptor for advanced glycation end products (RAGE)/NF-κB signaling pathway [81,82]. Epidemiological studies also indicate that long-term use of Aspirin or other non-steroidal anti-inflammatory drugs are protective against prostate cancer [83].
2.2. Crosstalk between NF-κB and Androgen Receptor Signaling
Just like the estrogen receptor and other steroid receptors are well-known inhibitors of NF-κB responses [84,85], the AR can also repress NF-κB-dependent transcription (Figure 2) [86,87]. In line with this, decreasing androgen levels or blocked AR signals are associated with elevated inflammatory markers [88,89].
AR specifically blocks canonical NF-κB (RelA/p50)-driven expression, but seems to positively induce non-canonical NF-κB activation (processing to mature p52) [90]. Activation of non-canonical NF-κB could thus be a very important step in the development of androgen independence, since loss of androgen repression of NF-κB target genes is associated with poor prognosis in metastatic prostate cancer [91]. The p52 subunit can also activate AR signaling, which, in addition to induction of metabolic reprogramming of prostate cancer cells through induction of genes for glucose uptake and metabolism, contributes to androgen-independent growth [63,92]. One mechanism for p52-induced androgen-independent AR signaling is the induced expression of a constitutively active AR splice form (AR-V7) [93,94]. On the other hand, the canonical IKKα and IKKβ upstream of NF-κB might also directly influence AR activity by phosphorylation [95], which might represent another level of cross-talk between NF-κB and AR.
Specific NF-κB subunits can also form heterodimers with AR. For example, an AR/p52 complex has been shown to be important for prostate cancer growth [96], an AR/c-Rel complex whose functional role is still unknown [11,97]. Also, high nuclear RelA, together with high nuclear AR expression, is associated with poor prostate cancer outcome, indicating the existence of other relevant NF-κB/AR interactions [98,99]. Combination treatments with pro-inflammatory stimuli and androgen has also revealed unique responses compared to each stimulation alone [100], indicating that NF-κB/AR complexes have specific functions and can induce specific transcriptional programs.
3. NF-κB Signaling Pathways in Prostate Cancers
3.1. Innate Immune Receptors in Prostate Cancer
Several innate immune receptors can be activated in the prostate as a consequence of infection or other stresses to cause prostate inflammation (prostatitis). In prostate cancer, the same receptors can also promote or worsen prostate cancer progression. The intracellular inflammasomes NLRP3, NLRP12 and AIM2 can be triggered by several stimuli, and are responsible for IL-1 and IL-18 expression in prostate cancer [101,102]. The lectin-like LOX-1 receptor, which detects oxidized low-density lipoprotein and advanced glycation endproducts (AGE), signals to NF-κB in prostate cancer and could represent another mechanism for comorbidities between metabolic syndrome and prostate cancer [103]. Another AGE receptor, RAGE, detects the danger signal/pro-inflammatory cytokine HMGB1, which plays an important role in prostate cancer metastasis development via NF-κB signaling [81]. The role of Toll-like receptors (TLRs), a major class of innate immune receptors, is a more complicated image. Different TLRs can either promote or inhibit prostate cancer growth [104]. Loss of the critical downstream TLR signaling component MyD88 significantly worsens the phenotypes in mouse models of prostate cancer, but these effects appear to be indirect and reflect the role of MyD88 in immune cells [105]. On the other hand, responses to viral signals via TLR3 or RIG-I, especially, seem to be highly detrimental to prostate cancer cells [106]. In line with this, prostate cancer cells can be very sensitive to interferons [107].
3.2. PKC and PKC-Related Signals in Prostate Cancer
One major class of oncogenic pro-inflammatory signaling proteins are members of the PKC family, which were discovered as the kinases responsible for the oncogenic effects of phorbol esters (i.e., PMA) [108]. Due to their common role in inflammation and cancer, PKCs are generally considered attractive therapeutic targets. For example, the broad range PKC inhibitor sotrastaurin has been used in clinical and pre-clinical trials, both as immunosuppressant [109,110] and cancer treatment [111]. Several PKCs have been implicated in prostate cancer with different downstream effects (Figure 3).
In prostate cancer, PKC activity is induced after AR inhibition, which could contribute to NF-κB-driven AR independence [112]. PKC-associated NF-κB responses in prostate cancer are specifically dependent on c-Rel, which induces transcripts associated with angiogenesis, inflammatory responses and cell motility [67]. PKCε is one upstream signaling activator of NF-κB in prostate cancer cells, which is often overexpressed in metastatic prostate cancer, and many studies argue for a causal link between PKCε overexpression and prostate cancer development [60,61,113,114]. Interestingly, it has been shown that the trypsin-activated proteinase-activated receptor (PAR)-2 signals via PKCε to induce cell proliferation, which could explain the pro-inflammatory effect of various secreted proteases from prostate cancer cells [115]. Recently, it was also discovered that PKCε acts synergistically with loss of PTEN (which results in constitutive Akt activation) for NF-κB activation, leading to elevated expression of the pro-inflammatory prostaglandin biosynthesis enzyme COX-2 [116].
In contrast, activation of other PKC isoforms, such as PKCα and PKCδ, by PMA stimulation of prostate cancer cells rather induces cell death due to autocrine TNF and TRAIL signaling [117]. This inflammation-induced autocrine suicide could theoretically be exploited as a therapy by blocking the pro-survival pathways downstream of TNF [118]. Interestingly, PKCδ expression in prostate cells is dependent on androgen signals, suggesting that therapeutic targeting of PKCδ might be highly dependent on the level of AR signaling in the prostate cancer.
Surprisingly, atypical PKCs, which require neither Ca2+ nor diacylglycerol for activation, seem to also play a role in NF-κB activation in the context of prostate cancer [119]. Expression of the atypical PKCλ or PKCι promotes hormone-independent growth of prostate cancer cells through the NF-κB dependent induction of pro-inflammatory proteins like IL-6, and genetic variants of PKCι have been associated with elevated prostate cancer risk [120,121]. IL-6, in turn, can induce expression of the anti-apoptotic Bcl-3 protein via STAT3 signaling, which can contribute to prostate cancer cell survival [20]. Knock-out of the prostate apoptosis response 4 (Par4), a pro-apoptotic tumor suppressor gene, results in spontaneous development of prostate cancer in mice, possibly due to loss of negative regulation of the atypical PKCζ and elevated expression of the anti-apoptotic protein XIAP [122]. Also, inhibition of PKCζ expression by Annexin A5 seems important in repressing COX-2 expression in prostate cancer cells [123]. This is in line with several observations showing that elevated expression and alternative splicing of PKCζ promotes an aggressive prostate cancer phenotype [124,125]. Of interest, the anti-rheumatic drug and atypical PKC inhibitor aurothiomalate is highly efficient against prostate cancer cells [126].
More distantly related members of the PKC superfamily like PKN1, PKN2 and PRKD3 (PKCυ) were shown to play an important role in prostate cancer motility [127,128], and inhibition of PKN1 has been shown to be an attractive therapeutic strategy [128,129]. On the other hand, another PKC superfamily member, PRKD1 (PKCµ), is inversely associated with prostate cancer malignancy [130], illustrating the complex role of the PKC superfamily in prostate cancer.
3.3. GPCR Signaling in Prostate Cancer
Several G protein-coupled receptors (GPCRs) are associated with poor prognosis in prostate cancer and might represent interesting pharmacological targets [131,132,133,134,135,136,137,138,139]. Many GPCRs that indicate poor prognosis of prostate cancer signal via the Gα12/Gα13—RhoA axis via PKC for NF-κB activation [140,141,142], but GPCRs also induce several other transcriptional regulators relevant for cancer, like AP-1, MRTF-A and YAP [140]. In agreement with this, parallel blocking of both the Akt and the NF-κB pathway seems to be important in eliminating the effect of hyperactive GPCR signaling in prostate cancer cells [143]. The most notable GPCR for prostate cancer is the thrombin receptor PAR-1, which is known to signal to NF-κB [144], and is also a well-known poor prognostic marker in prostate cancer [145,146,147,148]. Prostate cancer cells produce thrombotic extracellular vesicles, which can in turn activate the thrombin receptors on prostate cancer cells or surrounding stromal cells [149,150]. Low-dose thrombin inhibition has been suggested as a potential prostate cancer treatment [151]. Also, the anti-inflammatory drug Aspirin, which has been shown to have protective effects against prostate cancer [83], could in principle partially affect prostate cancer cells through its anti-thrombotic activity. The association of GPCR signaling with poor prognosis in prostate cancer is a common theme shared with other solid tumors like breast cancer [152]. Apart from dysregulation at the receptor level, activating mutations or overexpression of the Gα12, Gα13 and RhoA downstream signaling components were shown to be risk factors in prostate cancer [153,154,155]. Furthermore, RhoA has been shown to activate PKCζ, which in turn leads to enhanced cell proliferation [156]. Overexpression of other GPCR downstream signaling components like the guanine nucleotide exchange factor Vav3 in the prostate epithelium was also shown to be sufficient for induction of prostatitis and NF-κB-dependent prostate cancer development [157].
4. NF-κB-Induced Risk Factors in Prostate Cancer
The different NF-κB complexes induce a wide range of transcriptional responses, which in turn can contribute to prostate cancer malignancy. Targeting downstream effects could be very attractive as an alternative to broad inhibition of signaling pathways. In cancers, however, the concept of "upstream" and "downstream" is not always clear. For example, IKKε is a downstream target that is induced by NF-κB and inhibited by AR in prostate cancer cells, and AR-negative prostate cancer cells show constitutive IKKε activation [158]. IKKε, in turn, can induce an NF-κB-independent expression of IL-6, which contributes to malignancy and androgen independence [159]. IKKε could theoretically also contribute to prostate cancer malignancy by phosphorylating and inactivating tumor suppressors like CYLD [160,161]. Supporting the importance of IKKε, specific inhibitors have been shown to be potentially interesting therapeutic options in prostate cancer [162].
Apart from the well-described contribution of IL-6-induced responses to AR antagonist resistance [163], IL-1β can also contribute to the development of resistance by inducing a gene expression profile in androgen-dependent cells that resembles that of androgen-independent cells [164]. In addition to the role of inflammasomes in IL-1β production, elimination of PKCε in prostate cancer cells also blocks production of IL-1β [61]. IL-1β expression is high in AR-negative prostate cancer cells, but IL-1β also represses AR expression and promotes bone metastasis [165,166,167,168,169]. Interestingly, the IL-1 inhibitor Anakinra is able to suppress prostate cancer bone metastasis and could be an interesting therapeutic option [165]. Also, expression of RANK and its ligand by prostate cancer cells promotes epithelial-to-mesenchymal transition and bone metastasis [170,171], indicating a potential for RANK targeting in prostate cancer. In addition to IL-1β and IL-6, several other cytokines have been associated with prostate cancer. IL-30 (IL-27 p28 form) expression from prostate cancer cells is associated with advanced-grade prostate cancer and IL-30 stimulates prostate cancer cell proliferation in vitro [172]. IL-8 is expressed specifically in AR-independent cells, and expression of IL-8 in AR-dependent cells also induces AR independence [173]. IL-8 expression also confers resistance to cytotoxic chemotherapy in prostate cancer cells [174]. This pro-survival role is shared with IL-12, whose depletion by antibodies causes prostate cancer cell death via IFNγ in vivo [175]. Recently, also IL-23 produced by myeloid-derived suppressor cells was shown to activate the androgen receptor pathway in prostate tumor cells, promoting cell survival and proliferation in androgen deprived conditions [43]. Most interestingly, IL-23 blocking antibodies could restore sensitivity to androgen-deprivation therapy in mice.
Although TNF has been associated with cell death in stimulated prostate cancer cells in vitro [117,176], an in vivo model using TNF receptor 1 knock-out mice suggests, rather, that TNF promotes prostate cancer proliferation [177]. High prostatic expression of TNF has also been associated with poor clinical outcome [178]. To exploit this therapeutically, some pro-inflammatory cytokines such as TNF could perhaps be made to kill cytokine-producing prostate cancer cells, for example by combination treatments with IFNγ [107,179,180]. Not all pro-inflammatory cytokines are, however, beneficial for prostate cancer cell growth, survival and metastasis. IL-18 is produced in prostate cancer cells after IFNα treatment, and high IL-18 expression is associated with beneficial clinical effects [181,182]. In line with this and in contrast to IL-1β, overexpression of IL-18 in prostate cancer cells inhibits tumor growth in vivo [183]. Another indication for an important role of IL-18 in prostate cancer is that some genetic variants of the IL-18 promoter are associated with elevated prostate cancer risk in different populations [184,185,186]. Also, the pro-inflammatory cytokine IL-33 is preferentially lost in metastatic prostate cancer in order to avoid immune cell triggering [187]. Apart from the direct pro-inflammatory signals, chemokines can also be induced in prostate cancer cells or other cells by inflammatory cytokines, which in turn can promote migration of prostate cancer cells and bone metastasis [188,189,190].
The interaction between prostate cells and the immune microenvironment can be far more complex. Not only can the pro-inflammatory signals associated with activated ("M1") macrophages contribute to prostate cancer through enhanced proliferation and aggressiveness, anti-inflammatory cytokines (like IL-4) associated with alternatively activated ("M2") macrophages can also contribute to pathology by activating AR signaling [191]. Also, expression of IL-4 in androgen-dependent prostate cancer cells can induce androgen-independent growth [192]. In addition, IL-4 promotes the clonogenic expansion of prostate cancer stem-like cells [193]. On the other hand, genetic variants characterized by low production of IL-10 are associated with higher risk of prostate cancer recurrence [194], indicating that the anti-inflammatory role is dominant. It should be noted that many studies showing a role for specific cytokines are based on overexpression and await confirmation at more physiological expression levels. Also, many NF-κB activating signals and downstream factors can probably cause similar aggressive and AR-independent prostate cancer phenotypes, implying that it will be important to find those cytokines or mediators that play a non-redundant function in the promotion of aggressive prostate cancer in vivo.
5. Conclusions and Perspectives
Androgen deprivation therapy has become the main prostate cancer therapy for patients at different stages of disease. However, a considerable fraction of patients receiving such treatments ultimately progress to a more aggressive disease, developing androgen-independence. Acquiring a better understanding of the mechanisms that control the development of prostate cancer and resistance to androgen-deprivation therapy remains an unmet clinical need. There is significant positive and negative crosstalk between steroid hormone receptor signaling (including androgen receptors) and inflammatory signaling mediated by NF-κB and other transcription factors. In the context of therapeutic targeting, it is, therefore, highly interesting to identify central inflammatory signaling nodes that are responsible for the development of androgen independence. Up until recently, we have been limited to druggable targets in the signaling pathways as therapeutic options. Advances in genome editing (with for example CRISPR/Cas9) coupled with efficient and reliable targeting to prostate cancer cells will however open up for a wider selection of potential targets to manipulate therapeutically [195]. Also, several cytokines that have been implicated in prostate cancer (e.g., IL-1β, IL-6, IL-12, IL-23) and for which cytokine blocking antibodies are already in the clinic or under clinical evaluation for the treatment of autoimmune diseases, deserve to be clinically evaluated in men that have lethal prostate cancer. However, more general anti-inflammatory drugs could also be beneficial, in combination with other types of treatment. One such general anti-inflammatory drug that seems to stand out is Aspirin, which not only affects inflammatory signaling leading to NF-κB activation at several levels [80], but also shows additional therapeutic activities (like anti-thrombotic activity) and directly targets molecules like HMGB1 [82] and COX-2 [75], which have been shown to be important in prostate cancer. As with any disease mechanism studied mainly in mouse models, the prevalence and importance of certain risk factors in humans remains to be determined, in most cases. Patient stratification and predictive biomarkers will probably be crucial to successfully implement novel treatments in the clinic.
Noteworthy, prostate and breast cancers are in many ways similar [39], not only in that they both generally start off as sex hormone-dependent, but they also share mechanisms of inflammation-induced development of hormone independence. There is a striking overlap in pro-inflammatory signaling molecules leading to NF-κB activation and contributing to malignancy in both breast and prostate cancer (e.g., PKCε, IKKε and GPCRs). It might therefore be interesting to further explore these common themes on inflammation and sex hormone independence.
Author Contributions
J.S. and R.B. wrote the paper.
Funding
This research was funded by the Belgian Foundation against Cancer grant number FAF-F/2016/812.
Acknowledgments
APC was sponsored by MDPI.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Medicina. Available online: https://www.wdl.org/en/item/11618/ (accessed on 28 August 2018).
- Virchow, R. Die krankhaften Geschwülste: Strumen, Myome, Neurome, Angiome; Verlag Von August Hirschwald: Berlin, Germany, 1863. [Google Scholar]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Afonina, I.S.; Zhong, Z.; Karin, M.; Beyaert, R. Limiting inflammation-the negative regulation of NF-κB and the NLRP3 inflammasome. Nat. Immunol. 2017, 18, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Kochel, T.; Rice, N.R. v-rel- and c-rel-protein complexes bind to the NF-kappa B site in vitro. Oncogene 1992, 7, 567–572. [Google Scholar] [PubMed]
- Akram, N.; Imran, M.; Noreen, M.; Ahmed, F.; Atif, M.; Fatima, Z.; Bilal Waqar, A. Oncogenic Role of Tumor Viruses in Humans. Viral Immunol. 2017, 30, 20–27. [Google Scholar] [CrossRef] [PubMed]
- DiDonato, J.A.; Mercurio, F.; Karin, M. NF-κB and the link between inflammation and cancer. Immunol. Rev. 2012, 246, 379–400. [Google Scholar] [CrossRef] [PubMed]
- Clément, J.-F.; Meloche, S.; Servant, M.J. The IKK-related kinases: From innate immunity to oncogenesis. Cell Res. 2008, 18, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Wietek, C.; Cleaver, C.S.; Ludbrook, V.; Wilde, J.; White, J.; Bell, D.J.; Lee, M.; Dickson, M.; Ray, K.P.; O'Neill, L.A.J. IkappaB kinase epsilon interacts with p52 and promotes transactivation via p65. J. Biol. Chem. 2006, 281, 34973–34981. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.E.; Leslie, J.; Perkins, N.D. c-Rel and its many roles in cancer: An old story with new twists. Br. J. Cancer 2016, 114, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T.D.; Gerondakis, S. The c-Rel Transcription Factor in Development and Disease. Genes Cancer 2011, 2, 695–711. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hailfinger, S.; Nogai, H.; Pelzer, C.; Jaworski, M.; Cabalzar, K.; Charton, J.-E.; Guzzardi, M.; Décaillet, C.; Grau, M.; Dörken, B.; et al. Malt1-Dependent RelB Cleavage Promotes Canonical NF-κB Activation in Lymphocytes and Lymphoma Cell Lines. Proc. Natl. Acad. Sci. USA 2011, 108, 14596–14601. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.M.; Aleksiyadis, K.; Martin, A.; McNamee, K.; Tharmalingam, T.; Williams, R.O.; Mémet, S.; Cope, A.P. Inhibitor of kappa B epsilon (IκBε) is a non-redundant regulator of c-Rel-dependent gene expression in murine T and B cells. PLoS ONE 2011, 6, e24504. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.; Hayden, M.S.; Long, M.; Scott, M.L.; West, A.P.; Zhang, D.; Oeckinghaus, A.; Lynch, C.; Hoffmann, A.; Baltimore, D.; et al. IkappaBbeta acts to inhibit and activate gene expression during the inflammatory response. Nature 2010, 466, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- McKenna, S.; Wright, C.J. Inhibiting IκBβ-NFκB signaling attenuates the expression of select pro-inflammatory genes. J. Cell Sci. 2015, 128, 2143–2155. [Google Scholar] [CrossRef] [PubMed]
- Tsui, R.; Kearns, J.D.; Lynch, C.; Vu, D.; Ngo, K.A.; Basak, S.; Ghosh, G.; Hoffmann, A. IκBβ enhances the generation of the low-affinity NFκB/RelA homodimer. Nat. Commun. 2015, 6, 7068. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alexander, E.; Hildebrand, D.G.; Kriebs, A.; Obermayer, K.; Manz, M.; Rothfuss, O.; Schulze-Osthoff, K.; Essmann, F. IκBζ is a regulator of the senescence-associated secretory phenotype in DNA damage- and oncogene-induced senescence. J. Cell Sci. 2013, 126, 3738–3745. [Google Scholar] [CrossRef] [PubMed]
- Massoumi, R.; Chmielarska, K.; Hennecke, K.; Pfeifer, A.; Fässler, R. Cyld Inhibits Tumor Cell Proliferation by Blocking Bcl-3-Dependent NF-κB Signaling. Cell 2006, 125, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, V.; Melendez-Zajgla, J. Role of Bcl-3 in solid tumors. Mol. Cancer 2011, 10, 152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haas, G.P.; Delongchamps, N.; Brawley, O.W.; Wang, C.Y.; de la Roza, G. The Worldwide Epidemiology of Prostate Cancer: Perspectives from Autopsy Studies. Can. J. Urol. 2008, 15, 3866–3871. [Google Scholar] [PubMed]
- Biancardi, M.F.; dos Santos, F.C.A.; de Carvalho, H.F.; Sanches, B.D.A.; Taboga, S.R. Female prostate: Historical, developmental, and morphological perspectives. Cell Biol. Int. 2017, 41, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Risberg, G.; Johansson, E.E.; Hamberg, K. A theoretical model for analysing gender bias in medicine. Int. J. Equity Health 2009, 8, 28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pongtippan, A.; Malpica, A.; Levenback, C.; Deavers, M.T.; Silva, E.G. Skene's gland adenocarcinoma resembling prostatic adenocarcinoma. Int. J. Gynecol. Pathol. 2004, 23, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Leroy, B.E.; Northrup, N. Prostate cancer in dogs: Comparative and clinical aspects. Vet. J. Lond. Engl. 1997 2009, 180, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.K.; Elshafae, S.M.; Keller, E.T.; McCauley, L.K.; Rosol, T.J. Review of Animal Models of Prostate Cancer Bone Metastasis. Vet. Sci. 2014, 1, 16–39. [Google Scholar] [CrossRef]
- Di Sebastiano, K.M.; Pinthus, J.H.; Duivenvoorden, W.C.M.; Mourtzakis, M. Glucose impairments and insulin resistance in prostate cancer: The role of obesity, nutrition and exercise. Obes. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef] [PubMed]
- Conteduca, V.; Caffo, O.; Galli, L.; Maugeri, A.; Scarpi, E.; Maines, F.; Chiuri, V.E.; Lolli, C.; Kinspergher, S.; Schepisi, G.; et al. Association among metabolic syndrome, inflammation, and survival in prostate cancer. Urol. Oncol. 2018, 36, 240.e1–240.e11. [Google Scholar] [CrossRef] [PubMed]
- Shankar, E.; Vykhovanets, E.V.; Vykhovanets, O.V.; Maclennan, G.T.; Singh, R.; Bhaskaran, N.; Shukla, S.; Gupta, S. High-fat diet activates pro-inflammatory response in the prostate through association of Stat-3 and NF-κB. Prostate 2012, 72, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.; Liou, G.-Y. Macrophage Cytokines Enhance Cell Proliferation of Normal Prostate Epithelial Cells through Activation of ERK and Akt. Sci. Rep. 2018, 8, 7718. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ianni, M.; Porcellini, E.; Carbone, I.; Potenzoni, M.; Pieri, A.M.; Pastizzaro, C.D.; Benecchi, L.; Licastro, F. Genetic factors regulating inflammation and DNA methylation associated with prostate cancer. Prostate Cancer Prostatic Dis. 2013, 16, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Jerde, T.J.; Bushman, W. IL-1 induces IGF-dependent epithelial proliferation in prostate development and reactive hyperplasia. Sci. Signal. 2009, 2, ra49. [Google Scholar] [CrossRef] [PubMed]
- Penney, K.L.; Stampfer, M.J.; Jahn, J.L.; Sinnott, J.A.; Flavin, R.; Rider, J.R.; Finn, S.; Giovannucci, E.; Sesso, H.D.; Loda, M.; et al. Gleason Grade Progression Is Uncommon. Cancer Res. 2013, 73, 5163–5168. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bubendorf, L.; Schöpfer, A.; Wagner, U.; Sauter, G.; Moch, H.; Willi, N.; Gasser, T.C.; Mihatsch, M.J. Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Hum. Pathol. 2000, 31, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.; Zhang, Q.; Wang, S.; Zhang, D. The prognosis of different distant metastases pattern in prostate cancer: A population based retrospective study. Prostate 2018, 78, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Mokarram, P.; Alizadeh, J.; Razban, V.; Barazeh, M.; Solomon, C.; Kavousipour, S. Interconnection of Estrogen/Testosterone Metabolism and Mevalonate Pathway in Breast and Prostate Cancers. Curr. Mol. Pharmacol. 2017, 10, 86–114. [Google Scholar] [CrossRef] [PubMed]
- Risbridger, G.P.; Davis, I.D.; Birrell, S.N.; Tilley, W.D. Breast and prostate cancer: More similar than different. Nat. Rev. Cancer 2010, 10, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C. Endocrine-induced regression of cancers. Cancer Res. 1967, 27, 1925–1930. [Google Scholar] [CrossRef] [PubMed]
- Locke, J.A.; Guns, E.S.; Lubik, A.A.; Adomat, H.H.; Hendy, S.C.; Wood, C.A.; Ettinger, S.L.; Gleave, M.E.; Nelson, C.C. Androgen Levels Increase by Intratumoral De novo Steroidogenesis during Progression of Castration-Resistant Prostate Cancer. Cancer Res. 2008, 68, 6407–6415. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silva, J.A.F.; Bruni-Cardoso, A.; Augusto, T.M.; Damas-Souza, D.M.; Barbosa, G.O.; Felisbino, S.L.; Stach-Machado, D.R.; Carvalho, H.F. Macrophage roles in the clearance of apoptotic cells and control of inflammation in the prostate gland after castration. Prostate 2018, 78, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Calcinotto, A.; Spataro, C.; Zagato, E.; Mitri, D.D.; Gil, V.; Crespo, M.; Bernardis, G.D.; Losa, M.; Mirenda, M.; Pasquini, E.; et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature 2018, 1. [Google Scholar] [CrossRef] [PubMed]
- Austin, D.C.; Strand, D.W.; Love, H.L.; Franco, O.E.; Grabowska, M.M.; Miller, N.L.; Hameed, O.; Clark, P.E.; Matusik, R.J.; Jin, R.J.; et al. NF-κB and androgen receptor variant 7 induce expression of SRD5A isoforms and confer 5ARI resistance. Prostate 2016, 76, 1004–1018. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Turo, R.; Jallad, S.; Prescott, S.; Cross, W.R. Metastatic prostate cancer in transsexual diagnosed after three decades of estrogen therapy. Can. Urol. Assoc. J. 2013, 7, E544–E546. [Google Scholar] [CrossRef] [PubMed]
- Miksad, R.A.; Bubley, G.; Church, P.; Sanda, M.; Rofsky, N.; Kaplan, I.; Cooper, A. Prostate Cancer in a Transgender Woman 41 Years After Initiation of Feminization. JAMA 2006, 296, 2312–2317. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.C.A.; Leite, R.P.; Custódio, A.M.G.; Carvalho, K.P.; Monteiro-Leal, L.H.; Santos, A.B.; Góes, R.M.; Carvalho, H.F.; Taboga, S.R. Testosterone Stimulates Growth and Secretory Activity of the Female Prostate in the Adult Gerbil (Meriones unguiculatus). Biol. Reprod. 2006, 75, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Obiezu, C.V.; Giltay, E.J.; Magklara, A.; Scorilas, A.; Gooren, L.J.; Yu, H.; Howarth, D.J.; Diamandis, E.P. Serum and urinary prostate-specific antigen and urinary human glandular kallikrein concentrations are significantly increased after testosterone administration in female-to-male transsexuals. Clin. Chem. 2000, 46, 859–862. [Google Scholar] [PubMed]
- Aultman, B. Cisgender. TSQ Transgender Stud. Q. 2014, 1, 61–62. [Google Scholar] [CrossRef][Green Version]
- Helsen, C.; Van den Broeck, T.; Voet, A.; Prekovic, S.; Van Poppel, H.; Joniau, S.; Claessens, F. Androgen receptor antagonists for prostate cancer therapy. Endocr. Relat. Cancer 2014, 21, T105–T118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kregel, S.; Chen, J.L.; Tom, W.; Krishnan, V.; Kach, J.; Brechka, H.; Fessenden, T.B.; Isikbay, M.; Paner, G.P.; Szmulewitz, R.Z.; et al. Acquired resistance to the second-generation androgen receptor antagonist enzalutamide in castration-resistant prostate cancer. Oncotarget 2016, 7, 26259–26274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Colditz, J.; Rupf, B.; Maiwald, C.; Baniahmad, A. Androgens induce a distinct response of epithelial-mesenchymal transition factors in human prostate cancer cells. Mol. Cell. Biochem. 2016, 421, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, C.; Liu, F.; Li, H.-Z.; Peng, G.; Gao, X.; Dong, K.-Q.; Wang, H.-R.; Kong, D.-P.; Qu, M.; et al. Reciprocal Network between Cancer Stem-Like Cells and Macrophages Facilitates the Progression and Androgen Deprivation Therapy Resistance of Prostate Cancer. Clin. Cancer Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Debelec-Butuner, B.; Alapinar, C.; Varisli, L.; Erbaykent-Tepedelen, B.; Hamid, S.M.; Gonen-Korkmaz, C.; Korkmaz, K.S. Inflammation-mediated abrogation of androgen signaling: An in vitro model of prostate cell inflammation. Mol. Carcinog. 2014, 53, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Ammirante, M.; Luo, J.-L.; Grivennikov, S.; Nedospasov, S.; Karin, M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature 2010, 464, 302–305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wong, C.P.; Bray, T.M.; Ho, E. Induction of proinflammatory response in prostate cancer epithelial cells by activated macrophages. Cancer Lett. 2009, 276, 38–46. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharma, J.; Gray, K.P.; Harshman, L.C.; Evan, C.; Nakabayashi, M.; Fichorova, R.; Rider, J.; Mucci, L.; Kantoff, P.W.; Sweeney, C.J. Elevated IL-8, TNF-α, and MCP-1 in men with metastatic prostate cancer starting androgen-deprivation therapy (ADT) are associated with shorter time to castration-resistance and overall survival. Prostate 2014, 74, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.J.; Ruscetti, M.; Arenzana, T.L.; Tran, L.M.; Bianci-Frias, D.; Sybert, E.; Priceman, S.J.; Wu, L.; Nelson, P.S.; Smale, S.T.; et al. Pten null prostate epithelium promotes localized myeloid-derived suppressor cell expansion and immune suppression during tumor initiation and progression. Mol. Cell. Biol. 2014, 34, 2017–2028. [Google Scholar] [CrossRef] [PubMed]
- Verzella, D.; Fischietti, M.; Capece, D.; Vecchiotti, D.; Del Vecchio, F.; Cicciarelli, G.; Mastroiaco, V.; Tessitore, A.; Alesse, E.; Zazzeroni, F. Targeting the NF-κB pathway in prostate cancer: A promising therapeutic approach? Curr. Drug Targets 2016, 17, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Blando, J.; Perez, C.J.; Wang, H.; Benavides, F.J.; Kazanietz, M.G. Activation of nuclear factor κB (NF-κB) in prostate cancer is mediated by protein kinase C epsilon (PKCepsilon). J. Biol. Chem. 2012, 287, 37570–37582. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Uzquiza, A.; Lopez-Haber, C.; Jernigan, D.L.; Fatatis, A.; Kazanietz, M.G. PKCε Is an Essential Mediator of Prostate Cancer Bone Metastasis. Mol. Cancer Res. 2015, 13, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Longoni, N.; Sarti, M.; Albino, D.; Civenni, G.; Malek, A.; Ortelli, E.; Pinton, S.; Mello-Grand, M.; Ostano, P.; D'Ambrosio, G.; et al. ETS transcription factor ESE1/ELF3 orchestrates a positive feedback loop that constitutively activates NF-κB and drives prostate cancer progression. Cancer Res. 2013, 73, 4533–4547. [Google Scholar] [CrossRef] [PubMed]
- Nadiminty, N.; Lou, W.; Sun, M.; Chen, J.; Yue, J.; Kung, H.-J.; Evans, C.P.; Zhou, Q.; Gao, A.C. Aberrant activation of the androgen receptor by NF-kappaB2/p52 in prostate cancer cells. Cancer Res. 2010, 70, 3309–3319. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-H.; Park, S.-J.; Dickinson, S.I.; Luo, J.-L. A Constitutive Intrinsic Inflammatory Signaling Circuit Composed of miR-196b, Meis2, PPP3CC, and p65 Drives Prostate Cancer Castration Resistance. Mol. Cell 2017, 65, 154–167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, J.; Yi, S.; Zhou, J.; Zhang, Y.; Guo, F. The NF-κB subunit RelB regulates the migration and invasion abilities and the radio-sensitivity of prostate cancer cells. Int. J. Oncol. 2016, 49, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-C.; Qiu, T.; Dan, C.; Liu, X.-H.; Hu, C.-H. Blockage of RelB expression by gene silencing enhances the radiosensitivity of androgen-independent prostate cancer cells. Mol. Med. Rep. 2015, 11, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Caino, M.C.; Kazanietz, M.G. Regulation of Transcriptional Networks by PKC Isozymes: Identification of c-Rel as a Key Transcription Factor for PKC-Regulated Genes. PLoS ONE 2013, 8, e67319. [Google Scholar] [CrossRef] [PubMed]
- Ahlqvist, K.; Saamarthy, K.; Syed Khaja, A.S.; Bjartell, A.; Massoumi, R. Expression of Id proteins is regulated by the Bcl-3 proto-oncogene in prostate cancer. Oncogene 2013, 32, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. The cancer stem cell: Premises, promises and challenges. Nat. Med. 2011, 17, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Rajasekhar, V.K.; Studer, L.; Gerald, W.; Socci, N.D.; Scher, H.I. Tumour-initiating stem-like cells in human prostate cancer exhibit increased NF-κB signalling. Nat. Commun. 2011, 2, 162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nunes, J.J.; Pandey, S.K.; Yadav, A.; Goel, S.; Ateeq, B. Targeting NF-kappa B Signaling by Artesunate Restores Sensitivity of Castrate-Resistant Prostate Cancer Cells to Antiandrogens. Neoplasia 2017, 19, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Yamashita, H.; Yu, X.; Wang, J.; Franco, O.E.; Wang, Y.; Hayward, S.W.; Matusik, R.J. Inhibition of NF-kappa B signaling restores responsiveness of castrate-resistant prostate cancer cells to anti-androgen treatment by decreasing androgen receptor-variant expression. Oncogene 2015, 34, 3700–3710. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.J.; Lho, Y.; Connelly, L.; Wang, Y.; Yu, X.; Saint Jean, L.; Case, T.C.; Ellwood-Yen, K.; Sawyers, C.L.; Bhowmick, N.A.; et al. The nuclear factor-kappaB pathway controls the progression of prostate cancer to androgen-independent growth. Cancer Res. 2008, 68, 6762–6769. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, T.C.; van Aswegen, C.H.; du Plessis, D.J. Influence of acetylsalicylic acid and metabolites on DU-145 prostatic cancer cell proliferation. Oncology 1995, 52, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 1971, 231, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhou, W.; Ge, J.; Zhang, Z. Prostaglandin E2 receptor EP4 is involved in the cell growth and invasion of prostate cancer via the cAMP-PKA/PI3K-Akt signaling pathway. Mol. Med. Rep. 2018, 17, 4702–4712. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Lee, Y.-F.; Li, G.; Liu, S.; Bao, B.-Y.; Huang, J.; Hsu, C.-L.; Chang, C. A new prostate cancer therapeutic approach: Combination of androgen ablation with COX-2 inhibitor. Int. J. Cancer 2008, 123, 195–201. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ko, C.-J.; Lan, S.-W.; Lu, Y.-C.; Cheng, T.-S.; Lai, P.-F.; Tsai, C.-H.; Hsu, T.-W.; Lin, H.-Y.; Shyu, H.-Y.; Wu, S.-R.; et al. Inhibition of cyclooxygenase-2-mediated matriptase activation contributes to the suppression of prostate cancer cell motility and metastasis. Oncogene 2017, 36, 4597–4609. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; Tan, S.-H.; Sun, C.; Shaheduzzaman, S.; Hu, Y.; Petrovics, G.; Chen, Y.; Sesterhenn, I.A.; Li, H.; Sreenath, T.; et al. ERG oncogene modulates prostaglandin signaling in prostate cancer cells. Cancer Biol. Ther. 2011, 11, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Klessig, D.F.; Tian, M.; Choi, H.W. Multiple Targets of Salicylic Acid and Its Derivatives in Plants and Animals. Front. Immunol. 2016, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shao, S.; Han, D.; Xu, Y.; Jiao, D.; Wu, J.; Yang, F.; Ge, Y.; Shi, S.; Li, Y.; et al. High mobility group box 1 promotes the epithelial-to-mesenchymal transition in prostate cancer PC3 cells via the RAGE/NF-κB signaling pathway. Int. J. Oncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W.; Tian, M.; Song, F.; Venereau, E.; Preti, A.; Park, S.-W.; Hamilton, K.; Swapna, G.V.T.; Manohar, M.; Moreau, M.; et al. Aspirin's Active Metabolite Salicylic Acid Targets High Mobility Group Box 1 to Modulate Inflammatory Responses. Mol. Med. Camb. Mass 2015, 21, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Brusselaers, N. Maintenance use of aspirin or other non-steroidal anti-inflammatory drugs (NSAIDs) and prostate cancer risk. Prostate Cancer Prostatic Dis. 2018, 21, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Biswas, D.K.; Singh, S.; Shi, Q.; Pardee, A.B.; Iglehart, J.D. Crossroads of estrogen receptor and NF-kappaB signaling. Sci. STKE Signal Transduct. Knowl. Environ. 2005, 2005, pe27. [Google Scholar] [CrossRef]
- McKay, L.I.; Cidlowski, J.A. Cross-talk between nuclear factor-kappa B and the steroid hormone receptors: Mechanisms of mutual antagonism. Mol. Endocrinol. 1998, 12, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Nelius, T.; Filleur, S.; Yemelyanov, A.; Budunova, I.; Shroff, E.; Mirochnik, Y.; Aurora, A.; Veliceasa, D.; Xiao, W.; Wang, Z.; et al. Androgen receptor targets NFkappaB and TSP1 to suppress prostate tumor growth in vivo. Int. J. Cancer 2007, 121, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Palvimo, J.J.; Reinikainen, P.; Ikonen, T.; Kallio, P.J.; Moilanen, A.; Jänne, O.A. Mutual transcriptional interference between RelA and androgen receptor. J. Biol. Chem. 1996, 271, 24151–24156. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.; Basaria, S.; Ceda, G.P.; Ble, A.; Ling, S.M.; Bandinelli, S.; Valenti, G.; Ferrucci, L. The relationship between testosterone and molecular markers of inflammation in older men. J. Endocrinol. Investig. 2005, 28, 116–119. [Google Scholar]
- Zhang, B.; Kwon, O.-J.; Henry, G.; Malewska, A.; Wei, X.; Zhang, L.; Brinkley, W.; Zhang, Y.; Castro, P.D.; Titus, M.; et al. Non-Cell-Autonomous Regulation of Prostate Epithelial Homeostasis by Androgen Receptor. Mol. Cell 2016, 63, 976–989. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lessard, L.; Saad, F.; Le Page, C.; Diallo, J.-S.; Péant, B.; Delvoye, N.; Mes-Masson, A.-M. NF-kappaB2 processing and p52 nuclear accumulation after androgenic stimulation of LNCaP prostate cancer cells. Cell. Signal. 2007, 19, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Campa, V.M.; Baltziskueta, E.; Bengoa-Vergniory, N.; Gorroño-Etxebarria, I.; Wesołowski, R.; Waxman, J.; Kypta, R.M. A screen for transcription factor targets of glycogen synthase kinase-3 highlights an inverse correlation of NFκB and androgen receptor signaling in prostate cancer. Oncotarget 2014, 5, 8173–8187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cui, Y.; Nadiminty, N.; Liu, C.; Lou, W.; Schwartz, C.T.; Gao, A.C. Upregulation of glucose metabolism by NF-κB2/p52 mediates enzalutamide resistance in castration-resistant prostate cancer cells. Endocr. Relat. Cancer 2014, 21, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Nadiminty, N.; Tummala, R.; Liu, C.; Lou, W.; Evans, C.P.; Gao, A.C. NF-κB2/p52:c-Myc:hnRNPA1 Pathway Regulates Expression of Androgen Receptor Splice Variants and Enzalutamide Sensitivity in Prostate Cancer. Mol. Cancer Ther. 2015, 14, 1884–1895. [Google Scholar] [CrossRef] [PubMed]
- Nadiminty, N.; Tummala, R.; Liu, C.; Yang, J.; Lou, W.; Evans, C.P.; Gao, A.C. NF-κB2/p52 induces resistance to enzalutamide in prostate cancer: Role of androgen receptor and its variants. Mol. Cancer Ther. 2013, 12, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Jain, G.; Voogdt, C.; Tobias, A.; Spindler, K.-D.; Möller, P.; Cronauer, M.V.; Marienfeld, R.B. IκB kinases modulate the activity of the androgen receptor in prostate carcinoma cell lines. Neoplasia 2012, 14, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Mehraein-Ghomi, F.; Church, D.R.; Schreiber, C.L.; Weichmann, A.M.; Basu, H.S.; Wilding, G. Inhibitor of p52 NF-κB subunit and androgen receptor (AR) interaction reduces growth of human prostate cancer cells by abrogating nuclear translocation of p52 and phosphorylated AR(ser81). Genes Cancer 2015, 6, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, N.K.; Ferdinand, A.S.; Mukhopadhyay, L.; Cinar, B.; Lutchman, M.; Richie, J.P.; Freeman, M.R.; Liu, B.C.-S. Unraveling androgen receptor interactomes by an array-based method: Discovery of proto-oncoprotein c-Rel as a negative regulator of androgen receptor. Exp. Cell Res. 2006, 312, 3782–3795. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, L.; McCall, P.; Hatziieremia, S.; Catlow, J.; Adams, C.; McArdle, P.; Seywright, M.; Tanahill, C.; Paul, A.; Underwood, M.; et al. Nuclear factor κB predicts poor outcome in patients with hormone-naive prostate cancer with high nuclear androgen receptor. Hum. Pathol. 2012, 43, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Ide, H.; Mizushima, T.; Jiang, G.; Netto, G.J.; Gotoh, M.; Miyamoto, H. Nuclear Factor-κB Promotes Urothelial Tumorigenesis and Cancer Progression via Cooperation with Androgen Receptor Signaling. Mol. Cancer Ther. 2018. [Google Scholar] [CrossRef] [PubMed]
- Malinen, M.; Niskanen, E.A.; Kaikkonen, M.U.; Palvimo, J.J. Crosstalk between androgen and pro-inflammatory signaling remodels androgen receptor and NF-κB cistrome to reprogram the prostate cancer cell transcriptome. Nucleic Acids Res. 2017, 45, 619–630. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Fu, Y.; Tian, D.; Yan, W. The contrasting roles of inflammasomes in cancer. Am. J. Cancer Res. 2018, 8, 566–583. [Google Scholar] [PubMed]
- Karan, D.; Tawfik, O.; Dubey, S. Expression analysis of inflammasome sensors and implication of NLRP12 inflammasome in prostate cancer. Sci. Rep. 2017, 7, 4378. [Google Scholar] [CrossRef] [PubMed]
- Balzan, S.; Lubrano, V. LOX-1 receptor: A potential link in atherosclerosis and cancer. Life Sci. 2018, 198, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Y.; Zhang, Q.; Wang, F.; Zhang, D. Toll-like receptors and prostate cancer. Front. Immunol. 2014, 5, 352. [Google Scholar] [CrossRef] [PubMed]
- Peek, E.M.; Song, W.; Zhang, H.; Huang, J.; Chin, A.I. Loss of MyD88 leads to more aggressive TRAMP prostate cancer and influences tumor infiltrating lymphocytes. Prostate 2015, 75, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Palchetti, S.; Starace, D.; De Cesaris, P.; Filippini, A.; Ziparo, E.; Riccioli, A. Transfected poly(I:C) activates different dsRNA receptors, leading to apoptosis or immunoadjuvant response in androgen-independent prostate cancer cells. J. Biol. Chem. 2015, 290, 5470–5483. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Zeng, C.; Xie, J.; Alghamdi, N.J.; Song, Y.; Zhang, H.; Zhou, A.; Jin, D. Effects of interferons and double-stranded RNA on human prostate cancer cell apoptosis. Oncotarget 2015, 6, 39184–39195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Castagna, M.; Takai, Y.; Kaibuchi, K.; Sano, K.; Kikkawa, U.; Nishizuka, Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J. Biol. Chem. 1982, 257, 7847–7851. [Google Scholar] [PubMed]
- De Weerd, A.; Kho, M.; Kraaijeveld, R.; Zuiderwijk, J.; Weimar, W.; Baan, C. The protein kinase C inhibitor sotrastaurin allows regulatory T cell function. Clin. Exp. Immunol. 2014, 175, 296–304. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pascher, A.; De Simone, P.; Pratschke, J.; Salamé, E.; Pirenne, J.; Isoneimi, H.; Bijarnia, M.; Krishnan, I.; Klupp, J. Protein kinase C inhibitor sotrastaurin in de novo liver transplant recipients: A randomized phase II trial. Am. J. Transplant. 2015, 15, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, D.; Williams, K.; LaFollette, T.D.; Cannon, M.; Blachly, J.S.; Zhong, Y.; Woyach, J.A.; Williams, E.; Awan, F.T.; Jones, J.; et al. PKC-β as a therapeutic target in CLL: PKC inhibitor AEB071 demonstrates preclinical activity in CLL. Blood 2014, 124, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Yokomizo, A.; Takeuchi, A.; Kashiwagi, E.; Dejima, T.; Inokuchi, J.; Tatsugami, K.; Uchiumi, T.; Eto, M. Protein kinase C regulates Twist1 expression via NF-κB in prostate cancer. Endocr. Relat. Cancer 2017, 24, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Totoń, E.; Ignatowicz, E.; Skrzeczkowska, K.; Rybczyńska, M. Protein kinase Cε as a cancer marker and target for anticancer therapy. Pharmacol. Rep. 2011, 63, 19–29. [Google Scholar] [CrossRef]
- Garg, R.; Blando, J.M.; Perez, C.J.; Abba, M.C.; Benavides, F.; Kazanietz, M.G. Protein Kinase C Epsilon Cooperates with PTEN Loss for Prostate Tumorigenesis through the CXCL13-CXCR5 Pathway. Cell Rep. 2017, 19, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Myatt, A.; Hill, S.J. Trypsin stimulates the phosphorylation of p42,44 mitogen-activated protein kinases via the proteinase-activated receptor-2 and protein kinase C epsilon in human cultured prostate stromal cells. Prostate 2005, 64, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Blando, J.M.; Perez, C.J.; Lal, P.; Feldman, M.D.; Smyth, E.M.; Ricciotti, E.; Grosser, T.; Benavides, F.; Kazanietz, M.G. COX-2 mediates pro-tumorigenic effects of PKCε in prostate cancer. Oncogene 2018. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Gonzalez-Guerrico, A.; Kazanietz, M.G. PKC-mediated secretion of death factors in LNCaP prostate cancer cells is regulated by androgens. Mol. Carcinog. 2009, 48, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Pilling, A.B.; Hwang, O.; Boudreault, A.; Laurent, A.; Hwang, C. IAP Antagonists Enhance Apoptotic Response to Enzalutamide in Castration-Resistant Prostate Cancer Cells via Autocrine TNF-α Signaling. Prostate 2017, 77, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Win, H.Y.; Acevedo-Duncan, M. Atypical protein kinase C phosphorylates IKKalphabeta in transformed non-malignant and malignant prostate cell survival. Cancer Lett. 2008, 270, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, H.; Akimoto, K.; Nagashima, Y.; Kojima, Y.; Sasaki, T.; Ishiguro-Imagawa, Y.; Nakaigawa, N.; Ohno, S.; Kubota, Y.; Uemura, H. aPKClambda/iota promotes growth of prostate cancer cells in an autocrine manner through transcriptional activation of interleukin-6. Proc. Natl. Acad. Sci. USA 2009, 106, 16369–16374. [Google Scholar] [CrossRef] [PubMed]
- Campa, D.; Hüsing, A.; Stein, A.; Dostal, L.; Boeing, H.; Pischon, T.; Tjønneland, A.; Roswall, N.; Overvad, K.; Østergaard, J.N.; et al. Genetic variability of the mTOR pathway and prostate cancer risk in the European Prospective Investigation on Cancer (EPIC). PLoS ONE 2011, 6, e16914. [Google Scholar] [CrossRef] [PubMed][Green Version]
- García-Cao, I.; Duran, A.; Collado, M.; Carrascosa, M.J.; Martín-Caballero, J.; Flores, J.M.; Diaz-Meco, M.T.; Moscat, J.; Serrano, M. Tumour-suppression activity of the proapoptotic regulator Par4. EMBO Rep. 2005, 6, 577–583. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baek, H.-S.; Park, N.; Kwon, Y.-J.; Ye, D.-J.; Shin, S.; Chun, Y.-J. Annexin A5 suppresses cyclooxygenase-2 expression by downregulating the protein kinase C-ζ-nuclear factor-κB signaling pathway in prostate cancer cells. Oncotarget 2017, 8, 74263–74275. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Bee, A.; Brewer, D.; Dodson, A.; Beesley, C.; Ke, Y.; Ambroisine, L.; Fisher, G.; Møller, H.; Dickinson, T.; et al. PRKC-ζ Expression Promotes the Aggressive Phenotype of Human Prostate Cancer Cells and is a Novel Target for Therapeutic Intervention. Genes Cancer 2010, 1, 444–464. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Ireland, S.J.; Bee, A.; Beesley, C.; Forootan, S.S.; Dodson, A.; Dickinson, T.; Gerard, P.; Lian, L.-Y.; Risk, J.M.; et al. Splice variant PRKC-ζ(-PrC) is a novel biomarker of human prostate cancer. Br. J. Cancer 2012, 107, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Trani, M.; Sorrentino, A.; Busch, C.; Landström, M. Pro-apoptotic effect of aurothiomalate in prostate cancer cells. Cell Cycle 2009, 8, 306–313. [Google Scholar] [CrossRef] [PubMed][Green Version]
- He, J.-H.; Li, B.-X.; Han, Z.-P.; Zou, M.-X.; Wang, L.; Lv, Y.-B.; Zhou, J.-B.; Cao, M.-R.; Li, Y.-G.; Zhang, J.-Z. Snail-activated long non-coding RNA PCA3 up-regulates PRKD3 expression by miR-1261 sponging, thereby promotes invasion and migration of prostate cancer cells. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-S.; Melhuish, T.A.; Spencer, A.; Ni, L.; Hao, Y.; Jividen, K.; Harris, T.E.; Snow, C.; Frierson, H.F.; Wotton, D.; et al. The protein kinase C super-family member PKN is regulated by mTOR and influences differentiation during prostate cancer progression. Prostate 2017, 77, 1452–1467. [Google Scholar] [CrossRef] [PubMed]
- Jilg, C.A.; Ketscher, A.; Metzger, E.; Hummel, B.; Willmann, D.; Rüsseler, V.; Drendel, V.; Imhof, A.; Jung, M.; Franz, H.; et al. PRK1/PKN1 controls migration and metastasis of androgen-independent prostate cancer cells. Oncotarget 2014, 5, 12646–12664. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nickkholgh, B.; Sittadjody, S.; Rothberg, M.B.; Fang, X.; Li, K.; Chou, J.W.; Hawkins, G.A.; Balaji, K.C. Beta-catenin represses protein kinase D1 gene expression by non-canonical pathway through MYC/MAX transcription complex in prostate cancer. Oncotarget 2017, 8, 78811–78824. [Google Scholar] [CrossRef] [PubMed]
- Kaittanis, C.; Andreou, C.; Hieronymus, H.; Mao, N.; Foss, C.A.; Eiber, M.; Weirich, G.; Panchal, P.; Gopalan, A.; Zurita, J.; et al. Prostate-specific membrane antigen cleavage of vitamin B9 stimulates oncogenic signaling through metabotropic glutamate receptors. J. Exp. Med. 2017. [Google Scholar] [CrossRef]
- Rodriguez, M.; Siwko, S.; Liu, M. Prostate-Specific G-protein coupled receptor, an emerging biomarker regulating inflammation and prostate cancer invasion. Curr. Mol. Med. 2016, 16, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.S.; Lee, J.; Zhang, X.; Lindholm, P.F. Lysophosphatidic acid activates the RhoA and NF-κB through Akt/IκBα signaling and promotes prostate cancer invasion and progression by enhancing functional invadopodia formation. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 6775–6785. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.; Jung, Y.-J. Angiotensin II receptor blockers induce autophagy in prostate cancer cells. Oncol. Lett. 2017, 13, 3579–3585. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ling, M.-T.; Wang, X.; Lee, D.T.; Tam, P.C.; Tsao, S.-W.; Wong, Y.-C. Id-1 expression induces androgen-independent prostate cancer cell growth through activation of epidermal growth factor receptor (EGF-R). Carcinogenesis 2004, 25, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Yowell, C.W.; Daaka, Y. G protein-coupled receptors provide survival signals in prostate cancer. Clin. Prostate Cancer 2002, 1, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Silvertown, J.D.; Ng, J.; Sato, T.; Summerlee, A.J.; Medin, J.A. H2 relaxin overexpression increases in vivo prostate xenograft tumor growth and angiogenesis. Int. J. Cancer 2006, 118, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.; Ishiguro, H.; Teranishi, J.-I.; Yoshida, S.-I.; Umemura, S.; Kubota, Y.; Uemura, H. Regulation of androgen receptor expression through angiotensin II type 1 receptor in prostate cancer cells. Prostate 2011, 71, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Schuff-Werner, P.; Steiner, M. Thrombin/thrombin receptor (PAR-1)-mediated induction of IL-8 and VEGF expression in prostate cancer cells. Biochem. Biophys. Res. Commun. 2006, 343, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.M.; Brown, J.H. G Protein-Coupled Receptor and RhoA-Stimulated Transcriptional Responses: Links to Inflammation, Differentiation, and Cell Proliferation. Mol. Pharmacol. 2015, 88, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Loberg, R.D.; Tantivejkul, K.; Craig, M.; Neeley, C.K.; Pienta, K.J. PAR1-mediated RhoA activation facilitates CCL2-induced chemotaxis in PC-3 cells. J. Cell. Biochem. 2007, 101, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.L.; Mize, G.J.; Takayama, T.K. Protease-activated receptor mediated RhoA signaling and cytoskeletal reorganization in LNCaP cells. Biochemistry 2003, 42, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Vinall, R.L.; Mahaffey, C.M.; Davis, R.R.; Luo, Z.; Gandour-Edwards, R.; Ghosh, P.M.; Tepper, C.G.; de Vere White, R.W. Dual blockade of PKA and NF-κB inhibits H2 relaxin-mediated castrate-resistant growth of prostate cancer sublines and induces apoptosis. Horm. Cancer 2011, 2, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.-C.; Lin, M.-F. Protease-activated receptor 1: A role in prostate cancer metastasis. Clin. Prostate Cancer 2004, 3, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bastian, M.; Kohlschein, P.; Schuff-Werner, P.; Steiner, M. Expression of functional protease-activated receptor 1 in human prostate cancer cell lines. Urol. Res. 2003, 31, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, D.; Zhou, W.; Wang, M.; Xia, W.; Tang, Q. Prognostic value of matrix metalloprotease-1/protease-activated receptor-1 axis in patients with prostate cancer. Med. Oncol. 2014, 31. [Google Scholar] [CrossRef] [PubMed]
- Black, P.C.; Mize, G.J.; Karlin, P.; Greenberg, D.L.; Hawley, S.J.; True, L.D.; Vessella, R.L.; Takayama, T.K. Overexpression of protease-activated receptors-1,-2, and-4 (PAR-1, -2, and -4) in prostate cancer. Prostate 2007, 67, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, V.; Kohli, M.; Dennis, R.A.; Siegel, E.R.; Chiles, W.W.; Mukunyadzi, P. Thrombin receptor expression is upregulated in prostate cancer. Prostate 2006, 66, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Al Saleh, H.A.; Haas-Neill, S.; Al-Hashimi, A.; Kapoor, A.; Shayegan, B.; Austin, R.C.; Al-Nedawi, K. Thrombotic characteristics of extracellular vesicles derived from prostate cancer cells. Prostate 2018. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Mize, G.J.; Zhang, X.; Takayama, T.K. Kallikrein-related peptidase-4 initiates tumor-stroma interactions in prostate cancer through protease-activated receptor-1. Int. J. Cancer 2010, 126, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Nieman, M.T.; LaRusch, G.; Fang, C.; Zhou, Y.; Schmaier, A.H. Oral thrombostatin FM19 inhibits prostate cancer. Thromb. Haemost. 2010, 104, 1044–1048. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ekambaram, P.; Lee, J.-Y.L.; Hubel, N.E.; Hu, D.; Yerneni, S.; Campbell, P.G.; Pollock, N.; Klei, L.R.; Concel, V.J.; Delekta, P.C.; et al. The CARMA3-Bcl10-MALT1 Signalosome Drives NFκB Activation and Promotes Aggressiveness in Angiotensin II Receptor-Positive Breast Cancer. Cancer Res. 2018, 78, 1225–1240. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P. A Role for the G12 Family of Heterotrimeric G Proteins in Prostate Cancer Invasion. J. Biol. Chem. 2006, 281, 26483–26490. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, X.; Wang, J.; Wang, Y.; Dong, H.; Li, J. Genetic variants in RhoA and ROCK1 genes are associated with the development, progression and prognosis of prostate cancer. Oncotarget 2017, 8, 19298–19309. [Google Scholar] [CrossRef] [PubMed]
- El-Haibi, C.P.; Sharma, P.; Singh, R.; Gupta, P.; Taub, D.D.; Singh, S.; Lillard, J.W. Differential G protein subunit expression by prostate cancer cells and their interaction with CXCR5. Mol. Cancer 2013, 12, 64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ghosh, P.M.; Bedolla, R.; Mikhailova, M.; Kreisberg, J.I. RhoA-dependent murine prostate cancer cell proliferation and apoptosis: Role of protein kinase Czeta. Cancer Res. 2002, 62, 2630–2636. [Google Scholar] [PubMed]
- Liu, Y.; Mo, J.Q.; Hu, Q.; Boivin, G.; Levin, L.; Lu, S.; Yang, D.; Dong, Z.; Lu, S. Targeted overexpression of vav3 oncogene in prostatic epithelium induces nonbacterial prostatitis and prostate cancer. Cancer Res. 2008, 68, 6396–6406. [Google Scholar] [CrossRef] [PubMed]
- Péant, B.; Diallo, J.-S.; Lessard, L.; Delvoye, N.; Le Page, C.; Saad, F.; Mes-Masson, A.-M. Regulation of IkappaB kinase epsilon expression by the androgen receptor and the nuclear factor-kappaB transcription factor in prostate cancer. Mol. Cancer Res. 2007, 5, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Péant, B.; Gilbert, S.; Le Page, C.; Poisson, A.; L'Ecuyer, E.; Boudhraa, Z.; Bienz, M.N.; Delvoye, N.; Saad, F.; Mes-Masson, A.-M. IκB-Kinase-epsilon (IKKε) over-expression promotes the growth of prostate cancer through the C/EBP-β dependent activation of IL-6 gene expression. Oncotarget 2017, 8, 14487–14501. [Google Scholar] [CrossRef] [PubMed]
- Lork, M.; Kreike, M.; Staal, J.; Beyaert, R. Importance of Validating Antibodies and Small Compound Inhibitors Using Genetic Knockout Studies-T Cell Receptor-Induced CYLD Phosphorylation by IKKε/TBK1 as a Case Study. Front. Cell Dev. Biol. 2018, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Hutti, J.E.; Shen, R.R.; Abbott, D.W.; Zhou, A.Y.; Sprott, K.M.; Asara, J.M.; Hahn, W.C.; Cantley, L.C. Phosphorylation of the tumor suppressor CYLD by the breast cancer oncogene IKKε promotes cell transformation. Mol. Cell 2009, 34, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, J.; Jeong, J.-H.; Park, S.-J.; Wei, R.; Peng, J.; Luo, Z.; Chen, Y.T.; Feng, Y.; Luo, J.-L. Selective TBK1/IKKi dual inhibitors with anticancer potency. Int. J. Cancer 2014, 134, 1972–1980. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Liu, Q.; Liao, Q.; Wu, Q.; Sun, B.; Yang, Z.; Hu, X.; Tan, M.; Li, L. Interleukin-6/signal transducer and activator of transcription 3 promotes prostate cancer resistance to androgen deprivation therapy via regulating pituitary tumor transforming gene 1 expression. Cancer Sci. 2018, 109, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Jardin, S.E.; Kanchwala, M.S.; Jacob, J.; Merchant, S.; Meade, R.K.; Gahnim, N.M.; Nawas, A.F.; Xing, C.; Delk, N.A. Identification of an IL-1-induced gene expression pattern in AR+ PCa cells that mimics the molecular phenotype of AR- PCa cells. Prostate 2018, 78, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, K.; Shen, F.; Worrede-Mahdi, A.; Liu, Q.; Gong, Y.; Garcia, F.U.; Fatatis, A. Cooperation among heterogeneous prostate cancer cells in the bone metastatic niche. Oncogene 2017, 36, 2846–2856. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.A.; Patel, V.; Gwede, M.; Morgado, M.; Tomasevich, K.; Fong, E.L.; Farach-Carson, M.C.; Delk, N.A. IL-1β induces p62/SQSTM1 and represses androgen receptor expression in prostate cancer cells. J. Cell. Biochem. 2014, 115, 2188–2197. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Russell, M.R.; Shahriari, K.; Jernigan, D.L.; Lioni, M.I.; Garcia, F.U.; Fatatis, A. Interleukin-1β promotes skeletal colonization and progression of metastatic prostate cancer cells with neuroendocrine features. Cancer Res. 2013, 73, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Herroon, M.K.; Rajagurubandara, E.; Hardaway, A.L.; Powell, K.; Turchick, A.; Feldmann, D.; Podgorski, I. Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget 2013, 4, 2108–2123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Staverosky, J.A.; Zhu, X.-H.; Ha, S.; Logan, S.K. Anti-androgen resistance in prostate cancer cells chronically induced by interleukin-1β. Am. J. Clin. Exp. Urol. 2013, 1, 53–65. [Google Scholar] [PubMed]
- Chu, G.C.-Y.; Zhau, H.E.; Wang, R.; Rogatko, A.; Feng, X.; Zayzafoon, M.; Liu, Y.; Farach-Carson, M.C.; You, S.; Kim, J.; et al. RANK- and c-Met-mediated signal network promotes prostate cancer metastatic colonization. Endocr. Relat. Cancer 2014, 21, 311–326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, G.; Sircar, K.; Aprikian, A.; Potti, A.; Goltzman, D.; Rabbani, S.A. Expression of RANKL/RANK/OPG in primary and metastatic human prostate cancer as markers of disease stage and functional regulation. Cancer 2006, 107, 289–298. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Di Meo, S.; Airoldi, I.; Sorrentino, C.; Zorzoli, A.; Esposito, S.; Di Carlo, E. Interleukin-30 expression in prostate cancer and its draining lymph nodes correlates with advanced grade and stage. Clin. Cancer Res. 2014, 20, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.; Omori, Y.; Lyn, D.; Singh, R.K.; Meinbach, D.M.; Sandman, Y.; Lokeshwar, V.B.; Lokeshwar, B.L. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res. 2007, 67, 6854–6862. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Purcell, C.; Seaton, A.; Oladipo, O.; Maxwell, P.J.; O'Sullivan, J.M.; Wilson, R.H.; Johnston, P.G.; Waugh, D.J.J. Chemotherapy-induced CXC-chemokine/CXC-chemokine receptor signaling in metastatic prostate cancer cells confers resistance to oxaliplatin through potentiation of nuclear factor-kappaB transcription and evasion of apoptosis. J. Pharmacol. Exp. Ther. 2008, 327, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.; Roy, A.; Pahan, K. Selective neutralization of IL-12 p40 monomer induces death in prostate cancer cells via IL-12-IFN-γ. Proc. Natl. Acad. Sci. USA 2017, 114, 11482–11487. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Montgomery, R.B.; Schmidt, L.J.; Mostaghel, E.A.; Huang, H.; Nelson, P.S.; Tindall, D.J. Reduced tumor necrosis factor receptor-associated death domain expression is associated with prostate cancer progression. Cancer Res. 2009, 69, 9448–9456. [Google Scholar] [CrossRef] [PubMed]
- Galheigo, M.R.U.; Cruz, A.R.; Cabral, Á.S.; Faria, P.R.; Cordeiro, R.S.; Silva, M.J.B.; Tomiosso, T.C.; Gonçalves, B.F.; Pinto-Fochi, M.E.; Taboga, S.R.; et al. Role of the TNF-α receptor type 1 on prostate carcinogenesis in knockout mice. Prostate 2016, 76, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Berriguete, G.; Sánchez-Espiridión, B.; Cansino, J.R.; Olmedilla, G.; Martínez-Onsurbe, P.; Sánchez-Chapado, M.; Paniagua, R.; Fraile, B.; Royuela, M. Clinical significance of both tumor and stromal expression of components of the IL-1 and TNF-α signaling pathways in prostate cancer. Cytokine 2013, 64, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Vercammen, E.; Staal, J.; Van Den Broeke, A.; Haegman, M.; Vereecke, L.; Schotte, P.; Beyaert, R. Prolonged exposure to IL-1beta and IFNgamma induces necrosis of L929 tumor cells via a p38MAPK/NF-kappaB/NO-dependent mechanism. Oncogene 2008, 27, 3780–3788. [Google Scholar] [CrossRef] [PubMed]
- Shelke, G.V.; Jagtap, J.C.; Kim, D.-K.; Shah, R.D.; Das, G.; Shivayogi, M.; Pujari, R.; Shastry, P. TNF-α and IFN-γ Together Up-Regulates Par-4 Expression and Induce Apoptosis in Human Neuroblastomas. Biomedicines 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Ponomareva, L.; Liu, H.; Duan, X.; Dickerson, E.; Shen, H.; Panchanathan, R.; Choubey, D. AIM2, an IFN-inducible cytosolic DNA sensor, in the development of benign prostate hyperplasia and prostate cancer. Mol. Cancer Res. 2013, 11, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Lebel-Binay, S.; Thiounn, N.; De Pinieux, G.; Vieillefond, A.; Debré, B.; Bonnefoy, J.-Y.; Fridman, W.-H.; Pagès, F. IL-18 is produced by prostate cancer cells and secreted in response to interferons. Int. J. Cancer 2003, 106, 827–835. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tse, B.W.-C.; Russell, P.J.; Lochner, M.; Förster, I.; Power, C.A. IL-18 inhibits growth of murine orthotopic prostate carcinomas via both adaptive and innate immune mechanisms. PLoS ONE 2011, 6, e24241. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dwivedi, S.; Goel, A.; Khattri, S.; Mandhani, A.; Sharma, P.; Misra, S.; Pant, K.K. Genetic variability at promoters of IL-18 (pro-) and IL-10 (anti-) inflammatory gene affects susceptibility and their circulating serum levels: An explorative study of prostate cancer patients in North Indian populations. Cytokine 2015, 74, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Jurecekova, J.; Babusikova, E.; Kmetova Sivonova, M.; Drobkova, H.; Petras, M.; Kliment, J.; Halasova, E. Association between interleukin-18 variants and prostate cancer in Slovak population. Neoplasma 2017, 64, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.M.; Liu, J.N.; Wei, M.T.; He, Y.Z.; Zhou, Y.; Song, X.B.; Ying, B.W.; Huang, J. Effect of IL-18 gene promoter polymorphisms on prostate cancer occurrence and prognosis in Han Chinese population. Genet. Mol. Res. GMR 2013, 12, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Saranchova, I.; Han, J.; Huang, H.; Fenninger, F.; Choi, K.B.; Munro, L.; Pfeifer, C.; Welch, I.; Wyatt, A.W.; Fazli, L.; et al. Discovery of a Metastatic Immune Escape Mechanism Initiated by the Loss of Expression of the Tumour Biomarker Interleukin-33. Sci. Rep. 2016, 6, 30555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Urata, S.; Izumi, K.; Hiratsuka, K.; Maolake, A.; Natsagdorj, A.; Shigehara, K.; Iwamoto, H.; Kadomoto, S.; Makino, T.; Naito, R.; et al. C-C motif ligand 5 promotes migration of prostate cancer cells in the prostate cancer bone metastasis microenvironment. Cancer Sci. 2018, 109, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, J.; Xu, Y.; Koch, A.E.; Cai, Z.; Chen, X.; Galson, D.L.; Taichman, R.S.; Zhang, J. CXCL16 functions as a novel chemotactic factor for prostate cancer cells in vitro. Mol. Cancer Res. 2008, 6, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Schulze, J.; Weber, K.; Baranowsky, A.; Streichert, T.; Lange, T.; Spiro, A.S.; Albers, J.; Seitz, S.; Zustin, J.; Amling, M.; et al. p65-Dependent production of interleukin-1β by osteolytic prostate cancer cells causes an induction of chemokine expression in osteoblasts. Cancer Lett. 2012, 317, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Lou, W.; Nadiminty, N.; Lin, X.; Gao, A.C. Requirement for NF-(kappa)B in interleukin-4-induced androgen receptor activation in prostate cancer cells. Prostate 2005, 64, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Pinder, E.; Chun, J.Y.; Lou, W.; Sun, M.; Gao, A.C. Interleukin-4 stimulates androgen-independent growth in LNCaP human prostate cancer cells. Prostate 2008, 68, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Nappo, G.; Handle, F.; Santer, F.R.; McNeill, R.V.; Seed, R.I.; Collins, A.T.; Morrone, G.; Culig, Z.; Maitland, N.J.; Erb, H.H.H. The immunosuppressive cytokine interleukin-4 increases the clonogenic potential of prostate stem-like cells by activation of STAT6 signalling. Oncogenesis 2017, 6, e342. [Google Scholar] [CrossRef] [PubMed]
- Dluzniewski, P.J.; Wang, M.-H.; Zheng, S.L.; De Marzo, A.M.; Drake, C.G.; Fedor, H.L.; Partin, A.W.; Han, M.; Fallin, M.D.; Xu, J.; et al. Variation in IL10 and other genes involved in the immune response and in oxidation and prostate cancer recurrence. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2012, 21, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Takahashi, Y.; Narita, S.; Yang, Y.-C.; Li, X. Targeted delivery of CRISPR/Cas9 to prostate cancer by modified gRNA using a flexible aptamer-cationic liposome. Oncotarget 2016, 8, 9375–9387. [Google Scholar] [CrossRef] [PubMed]
Figure 1. Structure and signaling of NF-κB and NF-κB-associated components. (a) Domain composition of class I and II NF-κB family members. Rel homology domain (RHD), transactivating domain (TAD), leucine zipper (LZ), IκB ankyrin repeat domain is colored in orange (b) A simple overview of the canonical and non-canonical NF-κB signaling pathways. Kinases are presented in green, NF-κB subunits in red, and IκB proteins in orange. In both pathways, phosphorylation leads to the proteasomal removal of inhibitory domains (e.g., in case of p100) or proteins (in case of IκBα) that prevent the NF-κB dimers from entering the nucleus to activate transcription. Alternative canonical NF-κB activation pathways via IKKε/TBK1 or IKK-independent pathways are not shown.
Figure 1. Structure and signaling of NF-κB and NF-κB-associated components. (a) Domain composition of class I and II NF-κB family members. Rel homology domain (RHD), transactivating domain (TAD), leucine zipper (LZ), IκB ankyrin repeat domain is colored in orange (b) A simple overview of the canonical and non-canonical NF-κB signaling pathways. Kinases are presented in green, NF-κB subunits in red, and IκB proteins in orange. In both pathways, phosphorylation leads to the proteasomal removal of inhibitory domains (e.g., in case of p100) or proteins (in case of IκBα) that prevent the NF-κB dimers from entering the nucleus to activate transcription. Alternative canonical NF-κB activation pathways via IKKε/TBK1 or IKK-independent pathways are not shown.
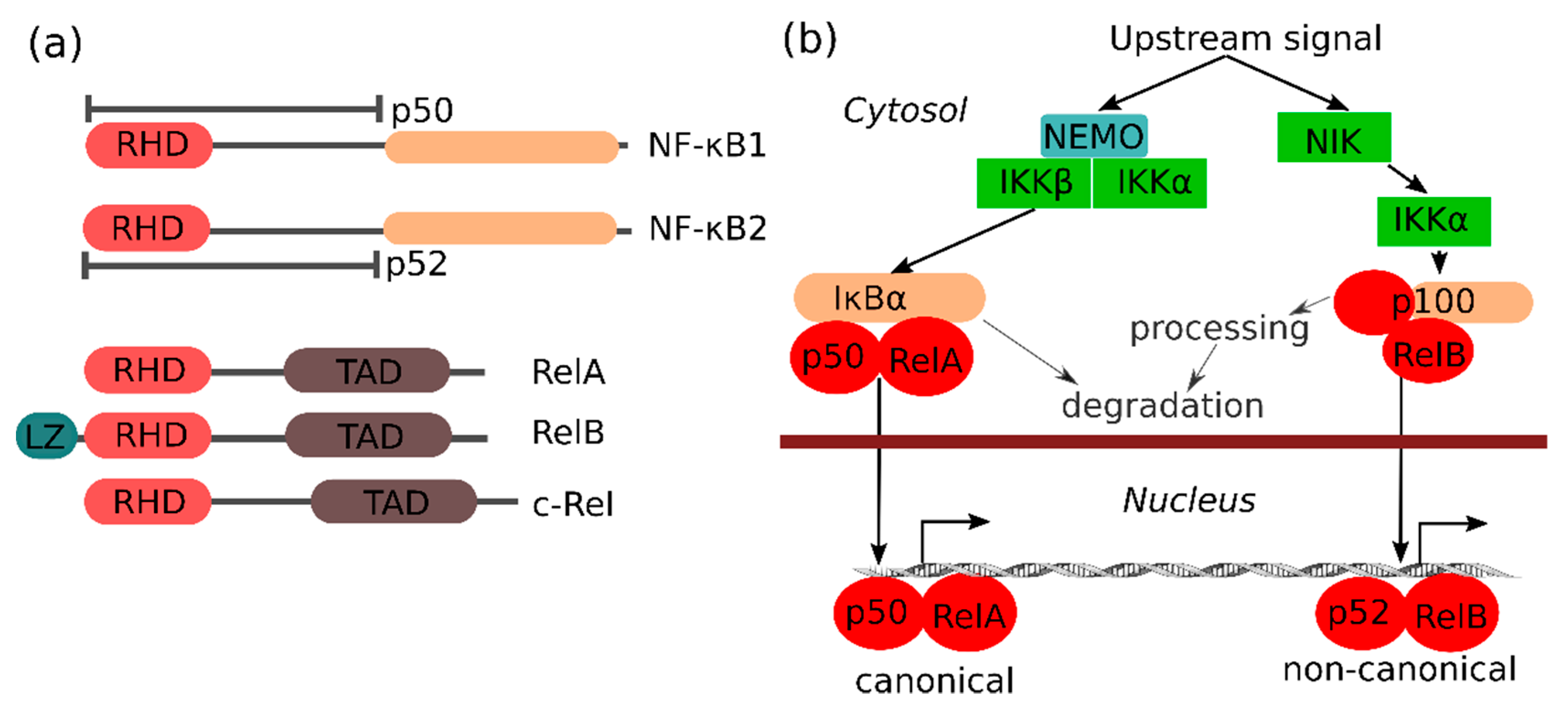
Figure 2. Schematic overview of crosstalk between AR and various NF-κB family members. Both p50/RelA and p52/RelB can drive AR expression, and p52/RelB can also drive expression of an androgen-independent AR splice variant (AR-V7). AR stimulation, in turn, represses canonical (p50/RelA) but promotes non-canonical (p52/RelB) NF-κB signaling. A potential feed-forward loop between AR and non-canonical NF-κB signaling is highlighted with purple arrows. AR can also act as a transactivator in complex with NF-κB family members, but the significance of the transcripts downstream of these hybrid transcription factors is currently unknown.
Figure 2. Schematic overview of crosstalk between AR and various NF-κB family members. Both p50/RelA and p52/RelB can drive AR expression, and p52/RelB can also drive expression of an androgen-independent AR splice variant (AR-V7). AR stimulation, in turn, represses canonical (p50/RelA) but promotes non-canonical (p52/RelB) NF-κB signaling. A potential feed-forward loop between AR and non-canonical NF-κB signaling is highlighted with purple arrows. AR can also act as a transactivator in complex with NF-κB family members, but the significance of the transcripts downstream of these hybrid transcription factors is currently unknown.
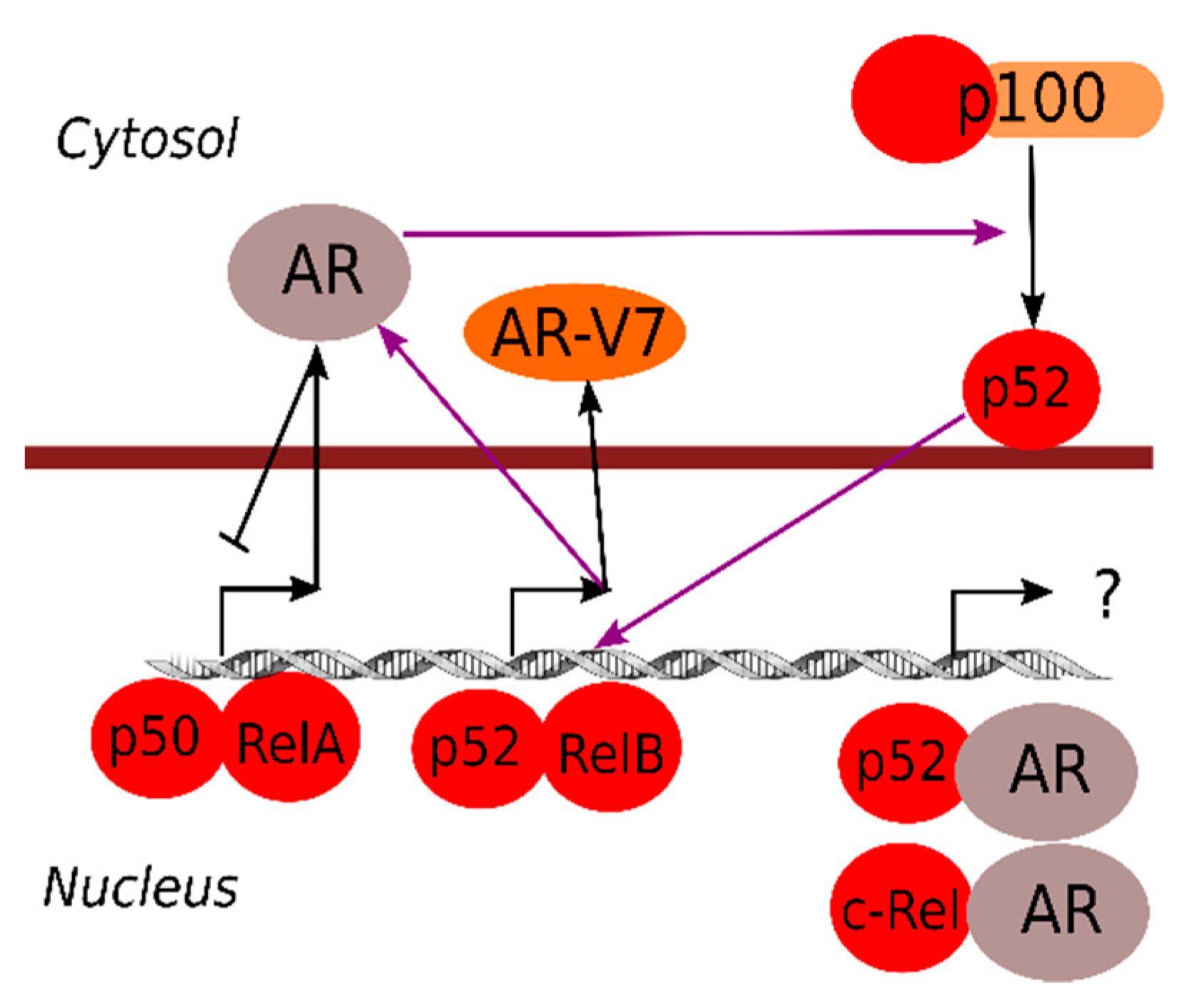
Figure 3. Overview of the role of PKC-dependent signaling in prostate cancer. PMA-induced activation of both PKCα and PKCδ has been associated with prostate cancer cell apoptosis due to autocrine TNF production, whereas elevated expression (arrow pointing up) of the other PKCs is associated with survival and aggressiveness of prostate cancer via multiple other mechanisms (see text for details). At least one of the pro-oncogenic PKCs (PKCε) can also be activated by upstream signaling.
Figure 3. Overview of the role of PKC-dependent signaling in prostate cancer. PMA-induced activation of both PKCα and PKCδ has been associated with prostate cancer cell apoptosis due to autocrine TNF production, whereas elevated expression (arrow pointing up) of the other PKCs is associated with survival and aggressiveness of prostate cancer via multiple other mechanisms (see text for details). At least one of the pro-oncogenic PKCs (PKCε) can also be activated by upstream signaling.
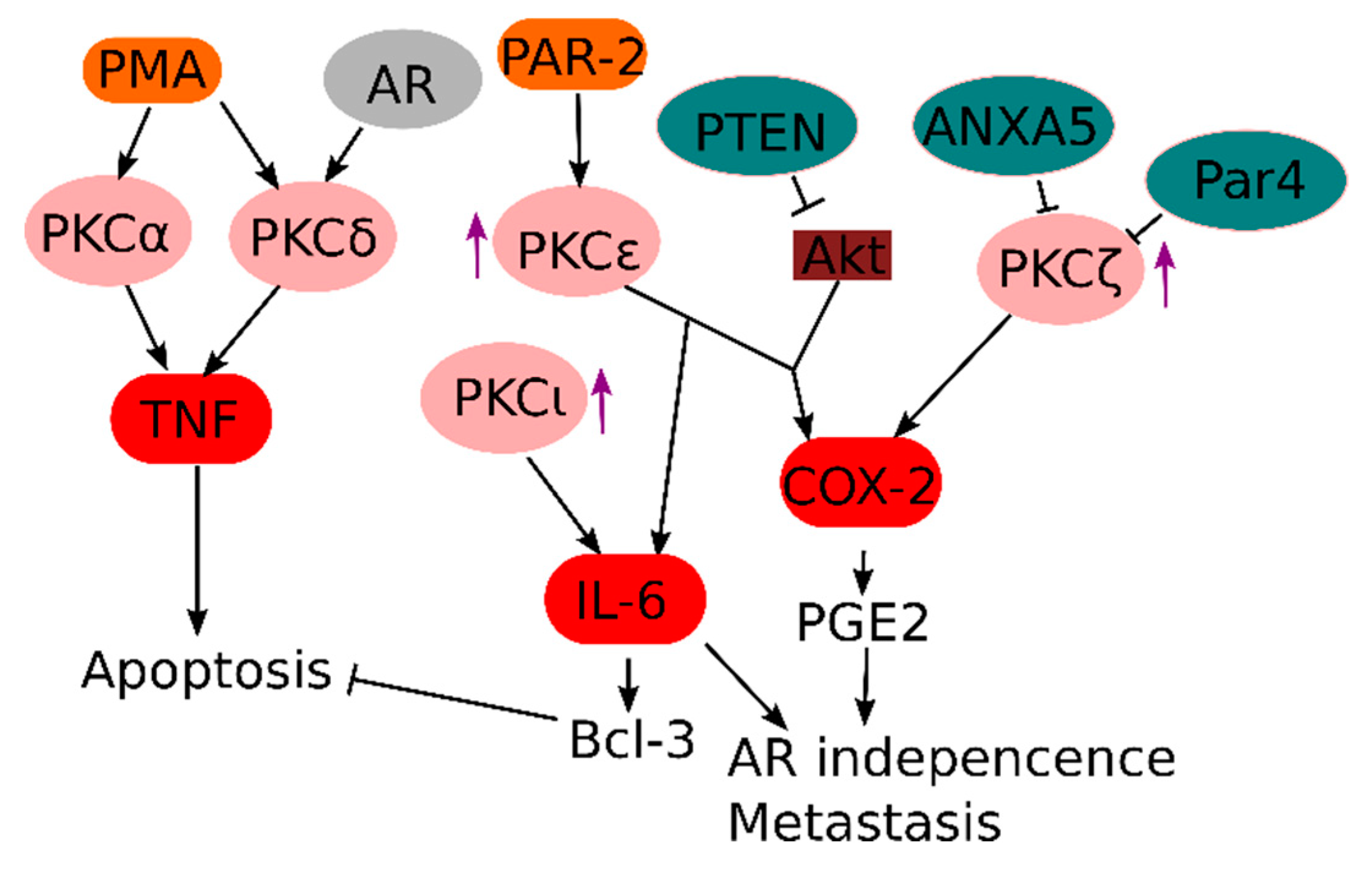
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Skene's Gland Prostate Change On Testosterone Ftm
Source: https://www.mdpi.com/2073-4409/7/9/122/htm

1 Komentar
We Rise GOC
BalasHapus